COVID-19 mRNA Vaccines: Lessons Learned from the Registrational Trials and Global Vaccination Campaign
Our understanding of COVID-19 vaccinations and their impact on health and mortality has evolved substantially since the first vaccine rollouts. Published reports from the original randomized phase 3 trials concluded that the COVID-19 mRNA vaccines ...

www.ncbi.nlm.nih.gov
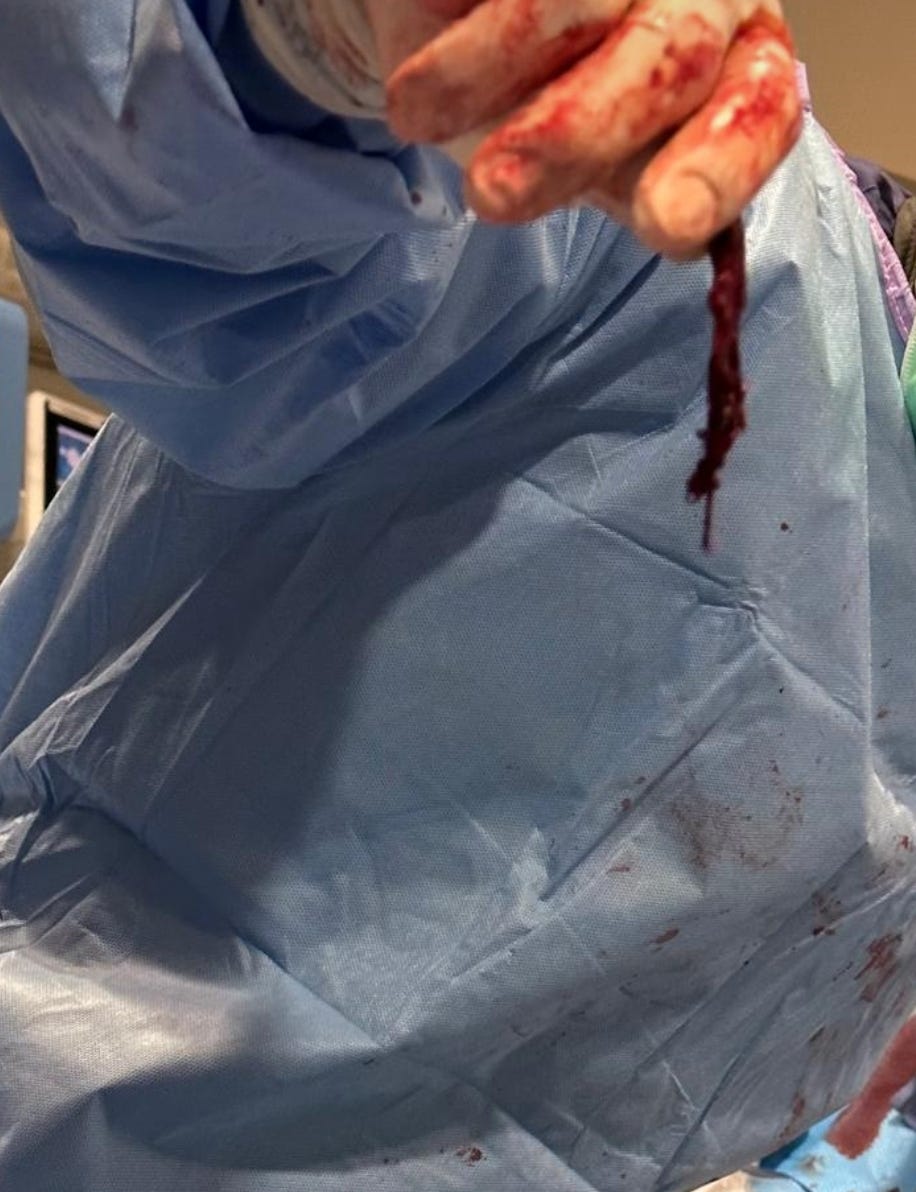
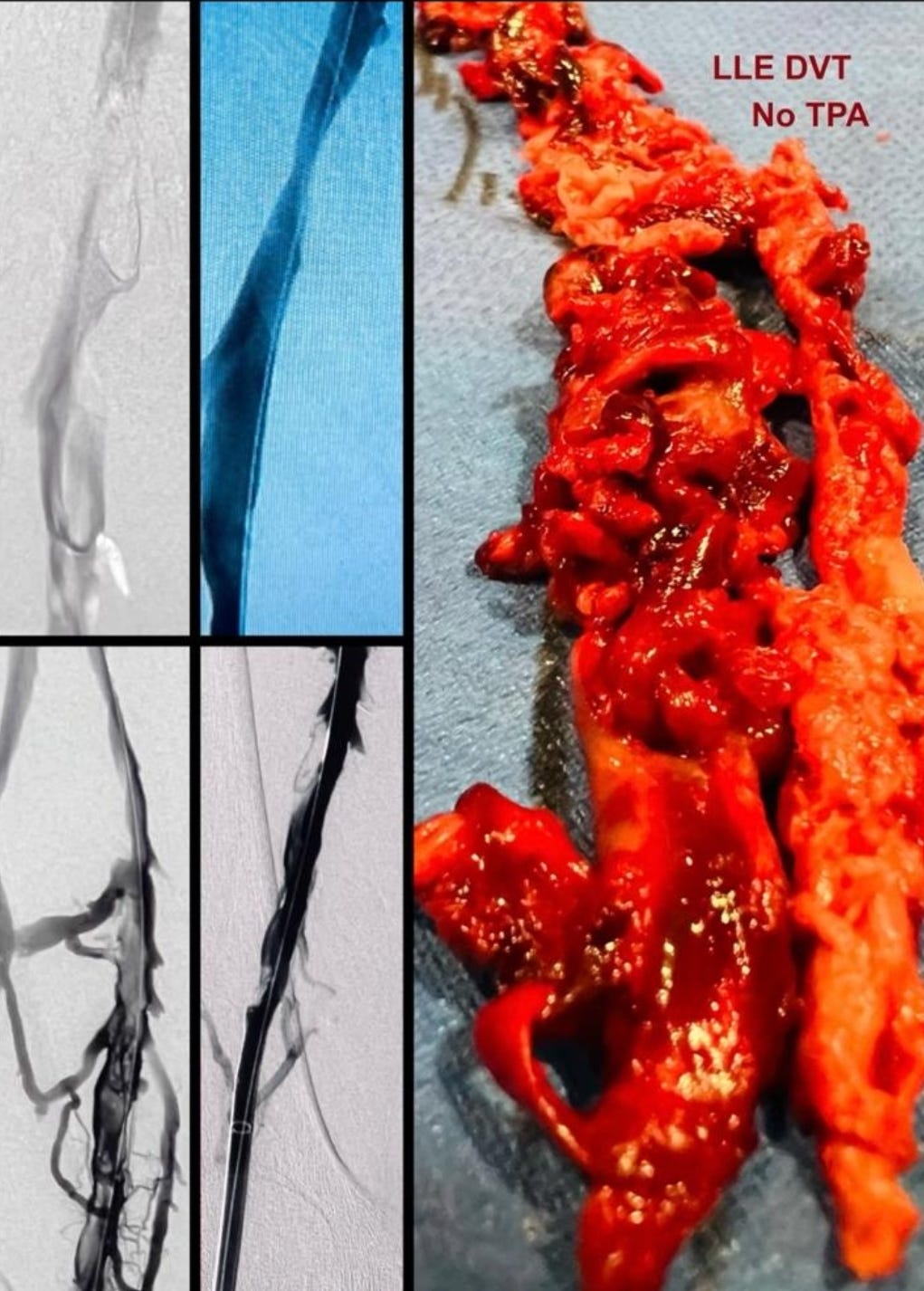
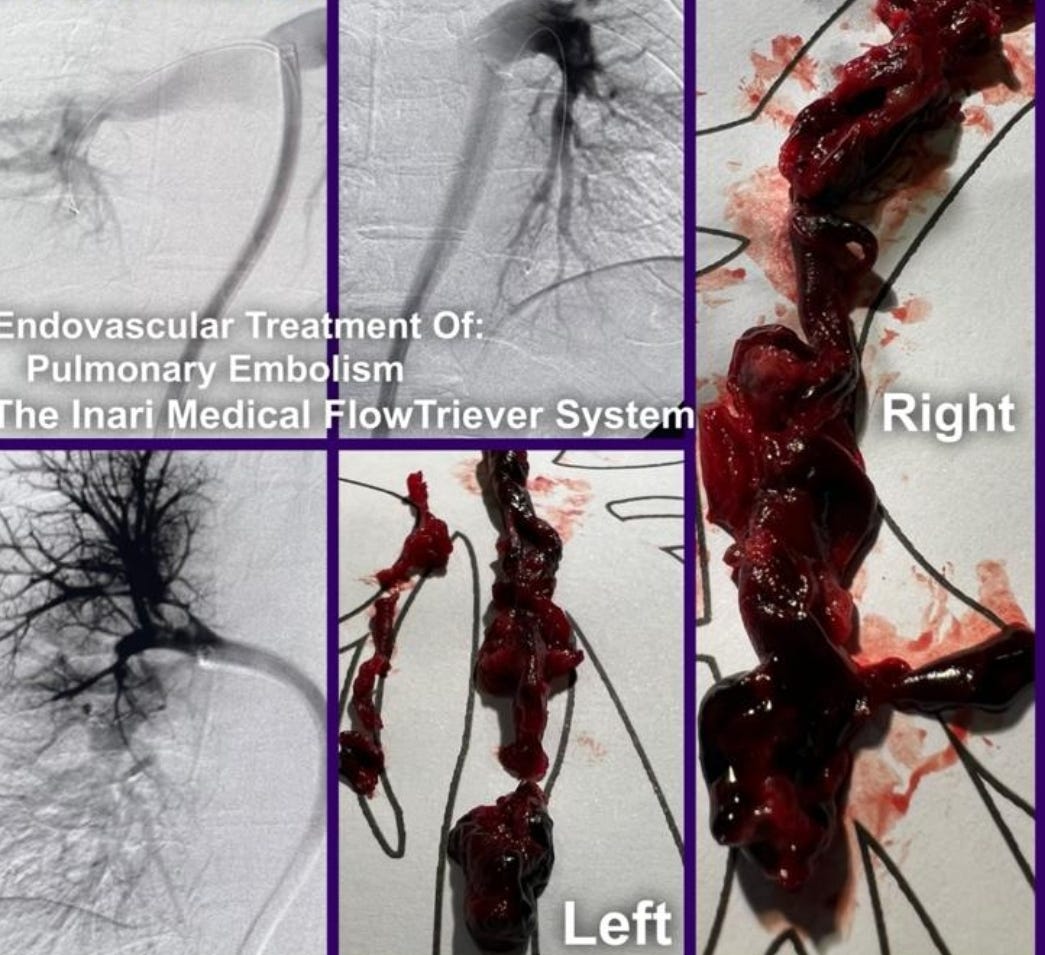
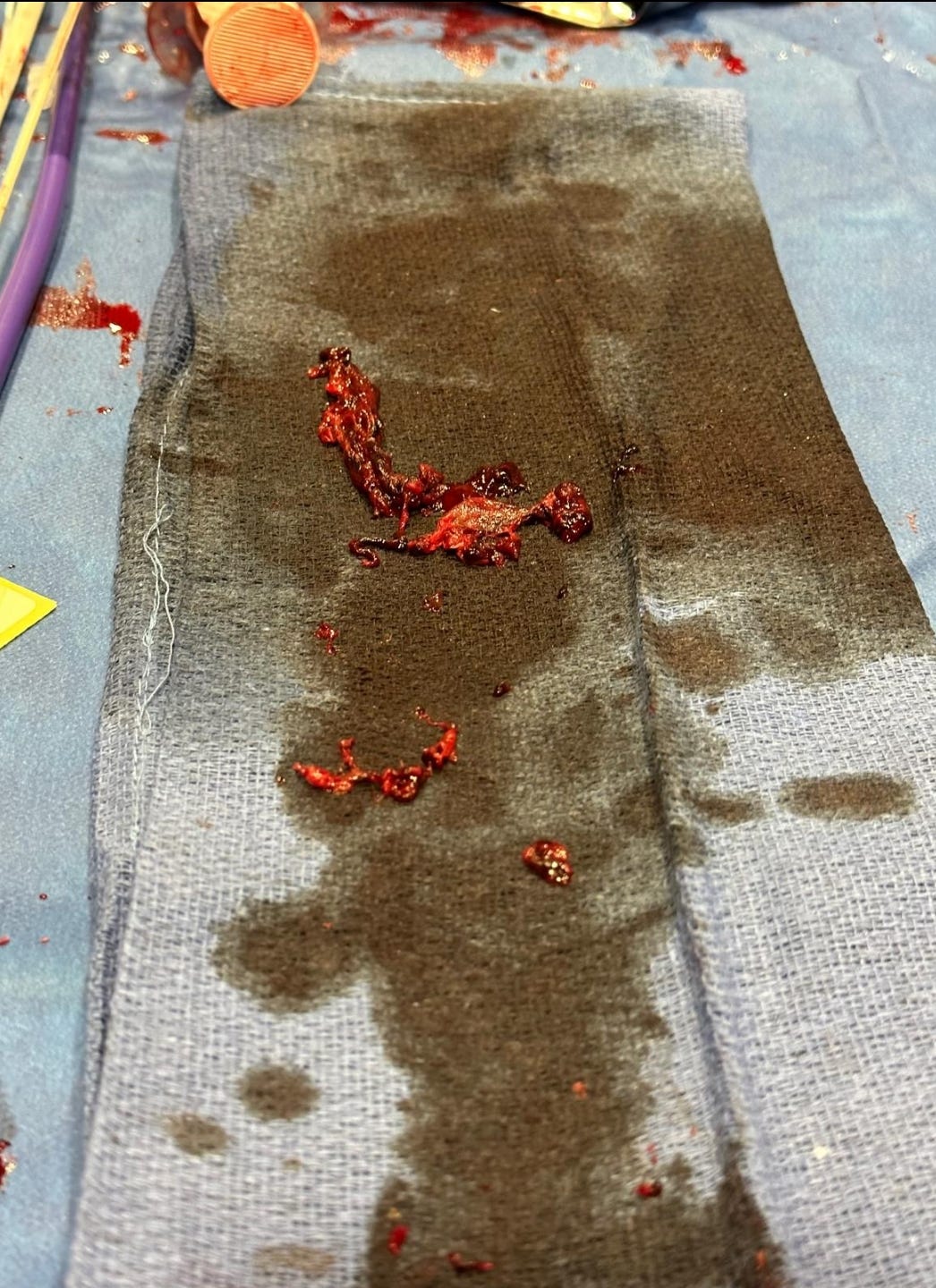
Re-analysis of the Pfizer trial data identified statistically significant increases in serious adverse events (SAEs) in the vaccine group. Numerous SAEs were identified following the Emergency Use Authorization (EUA), including
death, cancer, cardiac events, and various autoimmune, hematological, reproductive, and neurological disorders. Furthermore,
these products never underwent adequate safety and toxicological testing in accordance with previously established scientific standards. Among the other major topics addressed in this narrative review are the published analyses of serious harms to humans, quality control issues and process-related impurities, mechanisms underlying adverse events (AEs), the immunologic basis for vaccine inefficacy, and concerning mortality trends based on the registrational trial data.
 ec.europa.eu
ec.europa.eu